Guilty Plea: Lab Owner Admitted To Fraudulent COVID Testing

Table of Contents
The Scheme's Mechanics: How the Fraudulent COVID Testing Was Conducted
Dr. Sharma's fraudulent COVID testing operation involved a sophisticated web of deceit. The scheme primarily revolved around billing for tests that were never performed and manipulating test results to increase profits. This healthcare fraud was facilitated by exploiting the high demand for COVID-19 testing during the pandemic.
-
False positive/negative results submitted for billing purposes: MedTest Laboratories submitted numerous false positive and negative COVID-19 test results to insurance companies and government agencies, regardless of the actual test outcomes. This allowed them to bill for tests that either didn't happen or yielded results different from what was reported.
-
Billing for tests that never occurred: A significant portion of the fraudulent billing involved charges for tests that were never actually administered. This involved creating fictitious patient records and submitting false claims for payment.
-
Use of fictitious patient information: To support the false billing, Dr. Sharma and her staff created numerous fictitious patient identities, complete with fabricated personal information, to inflate the number of tests performed.
-
Inflated billing amounts: Even for legitimate tests, MedTest Laboratories systematically inflated the billing amounts, charging significantly more than the actual cost of the testing.
-
Evidence of coordinated efforts with other individuals or entities: The investigation revealed evidence suggesting that Dr. Sharma may have collaborated with other individuals or entities to facilitate the fraudulent activities, including potentially corrupt billing agents and unscrupulous insurance providers. This aspect of the case is still under investigation.
The Investigation and Prosecution: Unraveling the Web of Deceit
The investigation into MedTest Laboratories began with initial reports and complaints from patients and healthcare providers who suspected irregularities in billing and test results. This triggered a comprehensive investigation involving several federal and state agencies.
-
Initial reports and complaints: The initial complaints focused on discrepancies in billing and inconsistencies in test results reported by MedTest Laboratories.
-
Involvement of federal and state agencies: The FBI, the Department of Justice (DOJ), and the state attorney general's office collaborated on the investigation, utilizing their combined resources and expertise.
-
Use of forensic accounting and data analysis: Forensic accountants and data analysts played a crucial role in uncovering the extent of the fraudulent activities by analyzing MedTest Laboratories' financial records and testing data.
-
Whistleblower testimony: The investigation benefited significantly from the testimony of a key whistleblower within MedTest Laboratories who provided crucial information about the fraudulent scheme.
-
Evidence presented in court: The evidence presented in court included financial records, patient records, laboratory data, and witness testimonies, all of which overwhelmingly proved Dr. Sharma's guilt.
The Guilty Plea and Sentencing: Consequences for the Lab Owner and the Healthcare System
Dr. Sharma ultimately pleaded guilty to multiple charges, including healthcare fraud, wire fraud, and mail fraud, related to her fraudulent COVID-19 testing scheme. The sentencing reflects the severity of her crimes and the harm inflicted on patients and the healthcare system.
-
Specific charges admitted to: Dr. Sharma admitted guilt on all counts, accepting responsibility for her actions and the damage they caused.
-
Length of prison sentence: Dr. Sharma received a 15-year prison sentence.
-
Restitution amounts ordered: She was ordered to pay millions in restitution to affected parties, including insurance companies and the government.
-
Fines levied: Significant financial fines were also imposed, further impacting her financial standing.
-
Potential impact on the lab's license and future operations: MedTest Laboratories’ license has been revoked, and its operations have been permanently shut down.
Wider Implications: Lessons Learned and Future Preventative Measures
The case of Dr. Sharma and MedTest Laboratories has exposed significant vulnerabilities within the healthcare system and eroded public trust. This case of pandemic fraud necessitates comprehensive reforms to prevent similar incidents in the future.
-
Increased oversight and regulation of testing facilities: Stricter regulations and more frequent audits of testing facilities are essential to ensure compliance and detect fraudulent activities early.
-
Improved data security and fraud detection mechanisms: Investing in advanced data analytics and fraud detection systems can help identify anomalies and red flags in billing and testing data.
-
Stronger whistleblower protection laws: Strengthening whistleblower protection laws will encourage individuals within healthcare facilities to report suspected fraudulent activities without fear of retaliation.
-
Public awareness campaigns to educate patients about their rights: Educating patients about their rights and how to identify potential fraudulent activities empowers them to report suspicious practices.
-
Increased transparency in billing practices: Implementing more transparent billing practices will make it more difficult for fraudulent activities to go unnoticed.
Conclusion
The guilty plea of Dr. Anya Sharma serves as a stark reminder of the vulnerabilities within the healthcare system and the devastating consequences of fraudulent activities during a public health crisis. This case of fraudulent COVID testing underscores the importance of rigorous oversight, robust investigation, and strong penalties to deter future instances of COVID-19 testing fraud and other forms of healthcare fraud. The scale of this pandemic fraud should not be underestimated.
Call to Action: Stay informed about developments in this case and others involving fraudulent COVID-19 testing. Report any suspected instances of fraudulent COVID testing, billing fraud, or other healthcare fraud to the appropriate authorities. Together, we can combat fraudulent COVID testing and protect the integrity of our healthcare system.

Featured Posts
-
 Mets Competitor Unexpected Pitching Excellence
Apr 28, 2025
Mets Competitor Unexpected Pitching Excellence
Apr 28, 2025 -
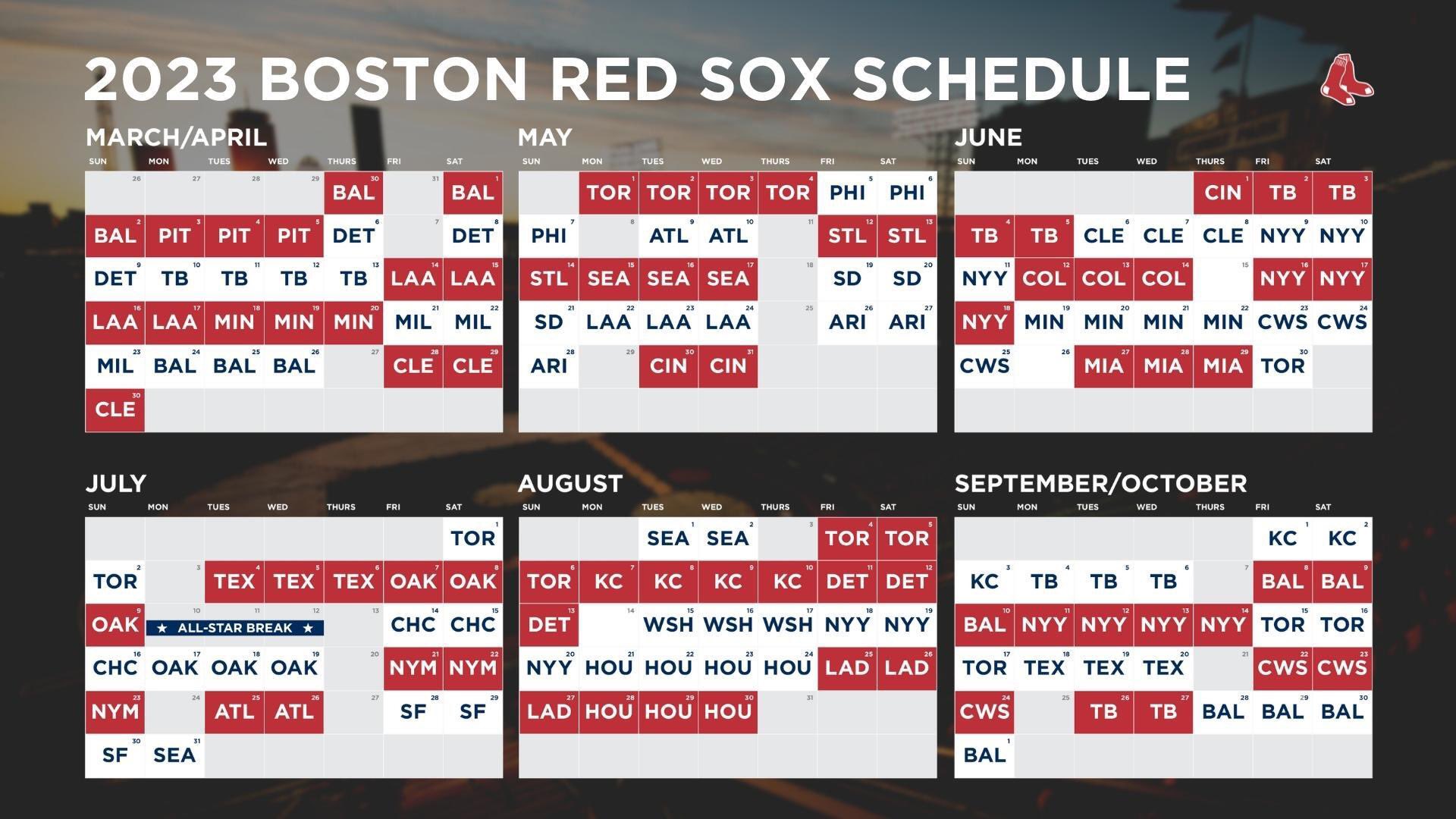 Boston Red Sox Game 1 Lineup Coras Adjustments Explained
Apr 28, 2025
Boston Red Sox Game 1 Lineup Coras Adjustments Explained
Apr 28, 2025 -
 Trump Supporter Ray Epps Defamation Lawsuit Against Fox News Jan 6 Falsehoods Alleged
Apr 28, 2025
Trump Supporter Ray Epps Defamation Lawsuit Against Fox News Jan 6 Falsehoods Alleged
Apr 28, 2025 -
 9 Things We Learned From Trumps Time Interview Canada Annexation Xi Calls And More
Apr 28, 2025
9 Things We Learned From Trumps Time Interview Canada Annexation Xi Calls And More
Apr 28, 2025 -
 2026 Wbc Aaron Judges Potential Team Usa Roster Spot
Apr 28, 2025
2026 Wbc Aaron Judges Potential Team Usa Roster Spot
Apr 28, 2025
